What is lemon pH level?

Are you wondering about the lemon pH level? If yes, then the answer is that Lemon juice or lemon has a pH level of 2.0.
What is pH?
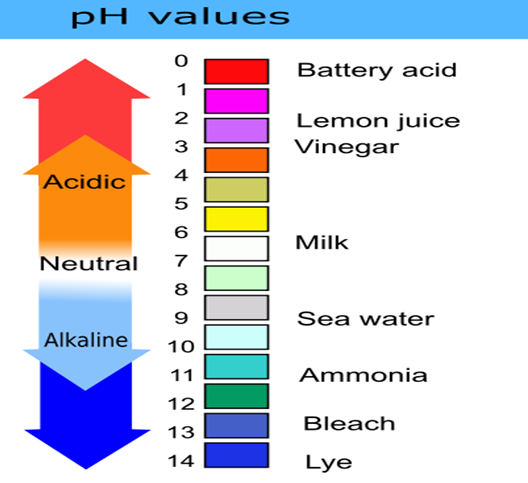
Power of hydrogen is abbreviated to pH. pH is a measure of how acidic or alkaline a solution is based on a scale of 0–14. In the pH scale, acidity and alkalinity are measured. The pH scale runs from 0 to 14; a pH of 7 is neutral, a pH below 7 is acidic, and a pH above 7 is alkaline.
Does the pH of food matter?
We eat food that passes through our gastrointestinal (GI) tract, which is actually considered to be outside of the body. With the help of other organs that secrete digestive enzymes, the gut breaks down food. Acidity or alkalinity of the original food is irrelevant by the time the digested food enters the blood stream. Blood pH is tightly regulated (by our own bodies) and is maintained in the range of 7.35 – 7.45, which is slightly alkaline. In order to maintain a balanced pH level in the body, extra acids or bases are excreted in the urine. Therefore, urine pH changes are completely normal and have no effect on blood pH. Hence we can say that body keeps your blood pH within a narrow, healthy range. Foods have little impact on blood pH.
Your doctor can better provide you with a better advice. We also offer free consultation. Book your appointment today.
Why lemon has acidic pH level?
Sometime we are wondering why the pH of lemon is acidic? Here’s the answer Lemons have an acidic pH because they contain citric acid in high quantities. A lemon juice’s pH ranges from 2 to 3, which means it is 10,000–100,000 times more acidic than water. Hence the pH of lemon is acidic due to the citric acid present in the lemon.
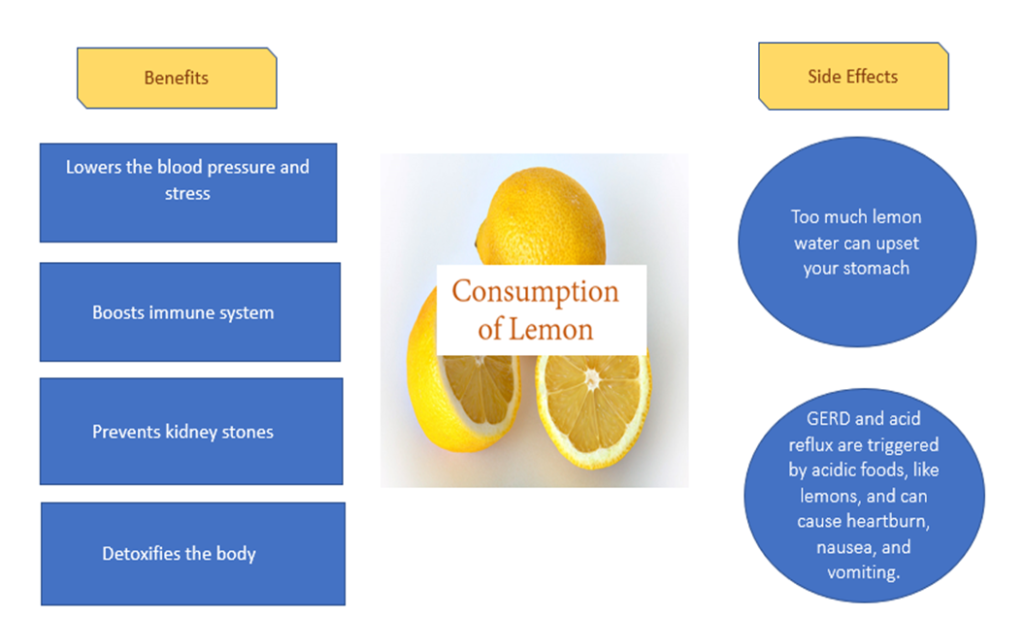
Advantages and Disadvantages of Consuming Lemons:
Everything in this world has pros and cons side by side. So, following are some of the advantages and disadvantages of consuming lemon:
Do you know strawberries are also acidic? Read more.
FAQ’s
Effect of drinking lemon water on your body pH level
Before it is digested, lemon juice has an acidic pH. Upon metabolization, it produces alkaline byproducts in the body. The alkaline byproducts in these foods can make your urine more alkaline but they have very little effect on your blood’s pH.
What fruit has the highest pH?
Below is a list of fruits and their pH from Clemson University. They are listed from most acidic to least acidic:
- Juice of lemon (pH: 2.00 to 2.60)
- Limes (pH: 2.00 to 2.80)
- Blue plums (pH: 2.80 to 3.40)
- Grapes (pH: 2.90 to 3.82)
- Pomegranates (pH: 2.93 to 3.20)
- Grapefruits(pH: 3.00 to 3.75)
- Blueberries (pH: 3.12 to 3.33)
- Pineapples (pH: 3.20 to 4.0)
- Apples (pH: 3.30 to 4.0)
- Peaches (pH: 3.30 to 4.05)
- Oranges (pH: 3.69–4.34)
- Tomatoes (pH: 4.30–4.90)
Does lemon raise or lower pH?
If you are thinking about whether lemon raises or lowers pH then the answer is that when lemon juice is added to water, it lowers pH.

